Humanity needs to improve when it comes to reducing carbon emissions to prevent the worst effects of climate change. If the world is to meet the IPCC's minimum target of keeping global temperature increases below 1.5 °C, every possible avenue for CO2 remediation must be explored.
Geological trapping can play a major role here. Our planet's underground rocks and sediments offer a vast potential space for long-term carbon storage. To support this, a recent computational study from a Japanese-led international group at Kyushu University shows how trapped carbon dioxide can be converted into harmless minerals.
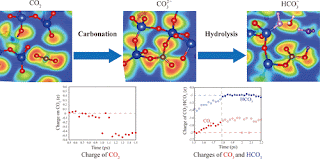
The rocks beneath the earth's surface are highly porous, and trapping involves injecting CO2 into the pores after collecting it from its emission source. Although CO2 is usually considered too stable to react chemically with rock, it can bind tightly to the surface by physical adsorption. Eventually it dissolves in water, forming carbonic acid, which can react with aqueous metals to form carbonate minerals.
"Mineralization is the most stable method of long-term CO2 storage, locking CO2 into a completely secure form that can't be re-emitted," explains Jihui Jia of the International Institute for Carbon-Neutral Energy Research (I2CNER), Kyushu University, first author of the study. "This was once thought to take thousands of years, but that view is rapidly changing. The chemical reactions are not fully understood because they're so hard to reproduce in the lab. This is where modeling comes in."
...
Evidence is growing that captured CO2 can mineralize much faster than previously believed. While this is exciting, the Kyushu paper underlines how complex and delicate the chemistry can be. For now, the group recommends further studies on other abundant rocks, like basalt, to map out the role that geochemical trapping can play in the greatest technical challenge facing civilization.
Read more at CO2 Mineralization in Geologically Common Rocks for Carbon Storage
No comments:
Post a Comment