An international team of researchers led by Bingqing Wei, a professor of mechanical engineering at the University of Delaware and the director of the Center for Fuel Cells and Batteries, is doing work that could lay the foundation for more widespread use of lithium metal batteries, which have more capacity than the lithium ion batteries commonly used in consumer electronics today. The team developed a method to mitigate dendrite formation in lithium metal batteries, which they have described in a paper published in Nano Letters.
The promise (and pitfalls) of lithium metal batteries
In a lithium ion battery, the anode, or current-generating side, is made of a material, such as graphite, with lithium ions bound to it. The lithium ions flow to the cathode, or current-collecting side.
In a lithium metal battery, the anode is made of lithium metal. Electrons flow from the anode to the cathode to generate electricity. Rechargeable batteries made of lithium metal hold a lot of promise because lithium is the most electrically positive metal and has a very high capacity.
“Theoretically, lithium metal is one of the best choices for batteries, but it is hard to handle in practice,” Wei said.
Lithium metal batteries have been inefficient, unstable, and even a fire hazard thus far. Their performance is hampered by lithium dendrites, formations that look like tiny stalagmites made of lithium deposits. As a battery is being used, lithium ions collect on the anode. Over time, the lithium deposits become non-uniform, leading to the formations of these dendrites, which can cause the battery to short circuit.
A new understanding
Research groups around the world have tried a variety of techniques to suppress the formation and growth of these dendrites. After studying the literature, Wei had found that almost all of the techniques applied could be understood under an umbrella: Introducing a layer of porous material into the system could deter dendrites from collecting on the anode.
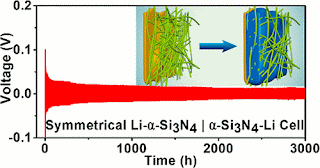
Using mathematical modeling, the research team found that a porous material suppressed the initiation and growth of dendrites. The dendrites that did form were 75 percent shorter than those that formed in systems that lacked the porous membrane. To further prove the finding, the team fabricated a membrane made of tiny wires of porous silicon nitride that measured less than one millionth of a meter each. They then integrated this membrane into lithium metal cells in a battery and ran it for 3,000 hours. No dendrites grew.
“This fundamental understanding may not be limited to the silicon nitride we used,” Wei said. “Other porous structures may do this too.”
What’s more, this principle may also extend to other battery systems, such as zinc- or potassium-based batteries, he said.
“In this field of metal-based batteries, this is up-to-date understanding,” he said. “This is the kind of work that could have high impact.”
Read more at Discovery Could Make Ultra-Powerful Batteries Safer, More Efficient
No comments:
Post a Comment