An international research team has engineered a molecule that uses light or electricity to convert the greenhouse gas carbon dioxide into carbon monoxide -- a carbon-neutral fuel source -- more efficiently than any other method of "carbon reduction." The discovery is a new milestone in the quest to recycle carbon dioxide in the Earth's atmosphere into carbon-neutral fuels and others materials.
The process is reported in the Journal of the American Chemical Society.
"If you can create an efficient enough molecule for this reaction, it will produce energy that is free and storable in the form of fuels," said Liang-shi Li, associate professor in the IU Bloomington College of Arts and Sciences' Department of Chemistry. "This study is a major leap in that direction."
Burning fuel -- such as carbon monoxide -- produces carbon dioxide and releases energy. Turning carbon dioxide back into fuel requires at least the same amount of energy. A major goal among scientists has been decreasing the excess energy needed.
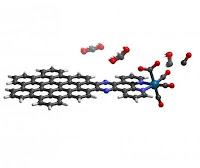
This is exactly what Li's molecule achieves: requiring the least amount of energy reported thus far to drive the formation of carbon monoxide. The molecule -- a nanographene-rhenium complex connected via an organic compound known as bipyridine -- triggers a highly efficient reaction that converts carbon dioxide to carbon monoxide.
The ability to efficiently and exclusively create carbon monoxide is significant due to the molecule's versatility.
"Carbon monoxide is an important raw material in a lot of industrial processes," Li said. "It's also a way to store energy as a carbon-neutral fuel since you're not putting any more carbon back into the atmosphere than you already removed. You're simply re-releasing the solar power you used to make it."
Read more at Chemists Create Molecular 'Leaf' that Collects and Stores Solar Power Without Solar Panels
No comments:
Post a Comment